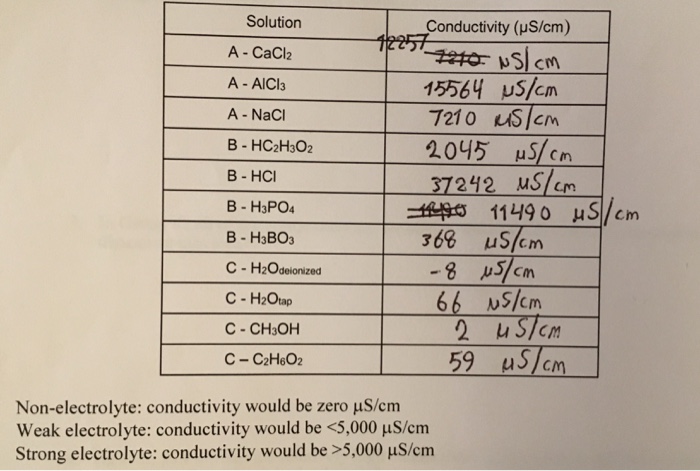
Therefore C 12 H 22 O 11 is non-electrolyte because it does not give ions with water. Solid CaCl2 is considered as an electrolyte because in when this solid is dissolved in water or any solution is made it is converted into ions which can conduct electricity.

Is Cacl2 Calcium Chloride An Electrolyte Or Non Electrolyte Youtube
Carbon tetrachloride neither dissociates into ions nor induces ion formation in the solvent.
. If its not an acid or base then it is a non. To tell if CaCl2 Calcium chloride is an electrolyte or nonelectrolyte we first need to know what type of compound we have. An electrolye is a material that causes ions charged entities to form in the solvent.
This solution is an electrolyte. CaCl2 C12H22O12 NH4Br Fe NO32 HC2H3O2 identify as soluble or insoluble in water. The compounds which when dissolved in water do not produce ions are called as non-electrolytes.
A compound that conducts an electric current when it is in an aqueous solution or melted. It is so weak that it is not even considered an electrolyte. So it is called an electrolyte even in solid state.
Because Ca is a metal and Cl is. I think the correct answer is option 3. Calcium chloride is used as an electrolyte in sports drinks.
Glucose commonly known as sugar dissolves readily in water but because it does not dissociate inside the solution into ions it is considered a nonelectrolyte. Nonelectrolytes are compounds that do not ionize at all in solution. Learn about its properties and uses.
Molecular compounds may be non-electrolytes weak electrolytes or strong electrolytes depending on whether they dissolve without ion formation a little ion. CaCl2 C12H22O12 NH4Br Fe NO32 HC2H3O2 identify as soluble. Typically nonelectrolytes are primarily held together by covalent rather than ionic bonds.
Chemistry questions and answers. If its molecular non-metal and non-metal take 2s considerations. If a substance is an ionic compound and strong acid then it is a strong electrolyte in water CaCl2 has a metal atom Ca and non metal atom Cl so it is an ionic compound and since it is ionic it will act as strong electrolyte.
Is the compound ionic non-metal and metal. A C 12 H 22 O 11. A common example of a nonelectrolyte is glucose or C 6 H 12 O 6.
Ie there is virtually no undissociated form of the compound in solution. If its ionic then it is a strong electrolyte. As a result solutions containing nonelectrolytes will not conduct electricity.
If it is molecular check if it is an acid or base. Steps to see if they are strong weak or non-electrolytes. Glucose a sugar with the chemical formula C6H12O6 is a typical example of a nonelectrolyte.
Identify as either a strong weak or non-electrolyte. An electrolyte is a substance that conducts electricity in solution. Therefore a sample of calcium chloride contains twice as many chloride ions as calcium ions.
Strong electrolytes because the small amounts that do dissolve in water do so principally as ions. When calcium chloride CaCl2 dissolves in water will the solution be an electrolyte or nonelectrolyte solution. The reason is that the C C l bond is rather strong and wont break under normal solution conditions.
Calcium Chloride when in solution dissociates into ions. A compound that does not conduct an electric current in either aqueous solution or in the molten state. In laboratories the drying tubes are usually packed with calcium chloride.
Identify as either a strong weak or non-electrolyte. CaSO4 LiF PbCl2 Sr OH2 Al NO33. It is a nonelectrolyte.
Solid CaCl2 does not conduct electricity explain. CaCl2 is the compound that is an electrolyte when dissolved in water. Calcium Chloride is an ionic compound with the chemical formula CaCl2.
Nonelectrolytes are usually held together by covalent bonds rather than ionic ones. To tell if BaCl2 Barium chloride is an electrolyte or nonelectrolyte we first need to know what type of compound we have. Because Ba is a metal and Cl is.
No C C l X 4 is not an electrolye. Is methanol a strong or weak electrolyte.
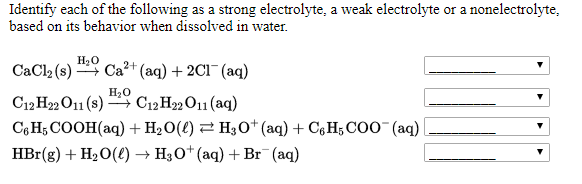
Solved Identify Each Of The Following As A Strong Chegg Com
Is Cacl2 Calcium Chloride An Electrolyte Or Non Electrolyte Youtube

Solved Solution A Cacl2 A Aici3 A Naci B Hc2h302 B Chegg Com

Is Cacl2 Calcium Chloride An Electrolyte Or Non Electrolyte Youtube
0 Comments